- Services
Technology Capabilities
Technology Capabilities- Product Strategy & Experience DesignDefine software-driven value chains, create purposeful interactions, and develop new segments and offerings.
- Digital Business TransformationAdvance your digital transformation journey.
- Intelligence EngineeringLeverage data and AI to transform products, operations, and outcomes.
- Software Product EngineeringCreate high-value products faster with AI-powered and human-driven engineering.
- Technology ModernizationTackle technology modernization with approaches that reduce risk and maximize impact.
- Embedded Engineering & IT/OT TransformationDevelop embedded software and hardware. Build IoT and IT/OT solutions.
- Industries
- GlobalLogic VelocityAI
- Insights
BlogsDecember 16, 2024Gene LeybzonAccelerating Digital Transformation with Structured AI Outputs
This code produces the following output that can be imported into the candidate trackin...
 BlogsOctober 30, 2024Yuriy Yuzifovich
BlogsOctober 30, 2024Yuriy YuzifovichAccelerating Enterprise Value with AI
Discover how financial services integrations are transforming from standalone offerings...
- About Us
Press ReleaseGlobalLogicMarch 11, 2025GlobalLogic Launches VelocityAI to Harness the Power of AI, ...
VelocityAI combines advanced AI technologies with human expertise, helping businesses r...
 Press ReleaseGlobalLogicJanuary 10, 2025
Press ReleaseGlobalLogicJanuary 10, 2025GlobalLogic Announces Leadership Change: Srini Shankar Appointed ...
SANTA CLARA, Calif.–January 10, 2025– GlobalLogic Inc., a Hitachi Group Com...

- Careers
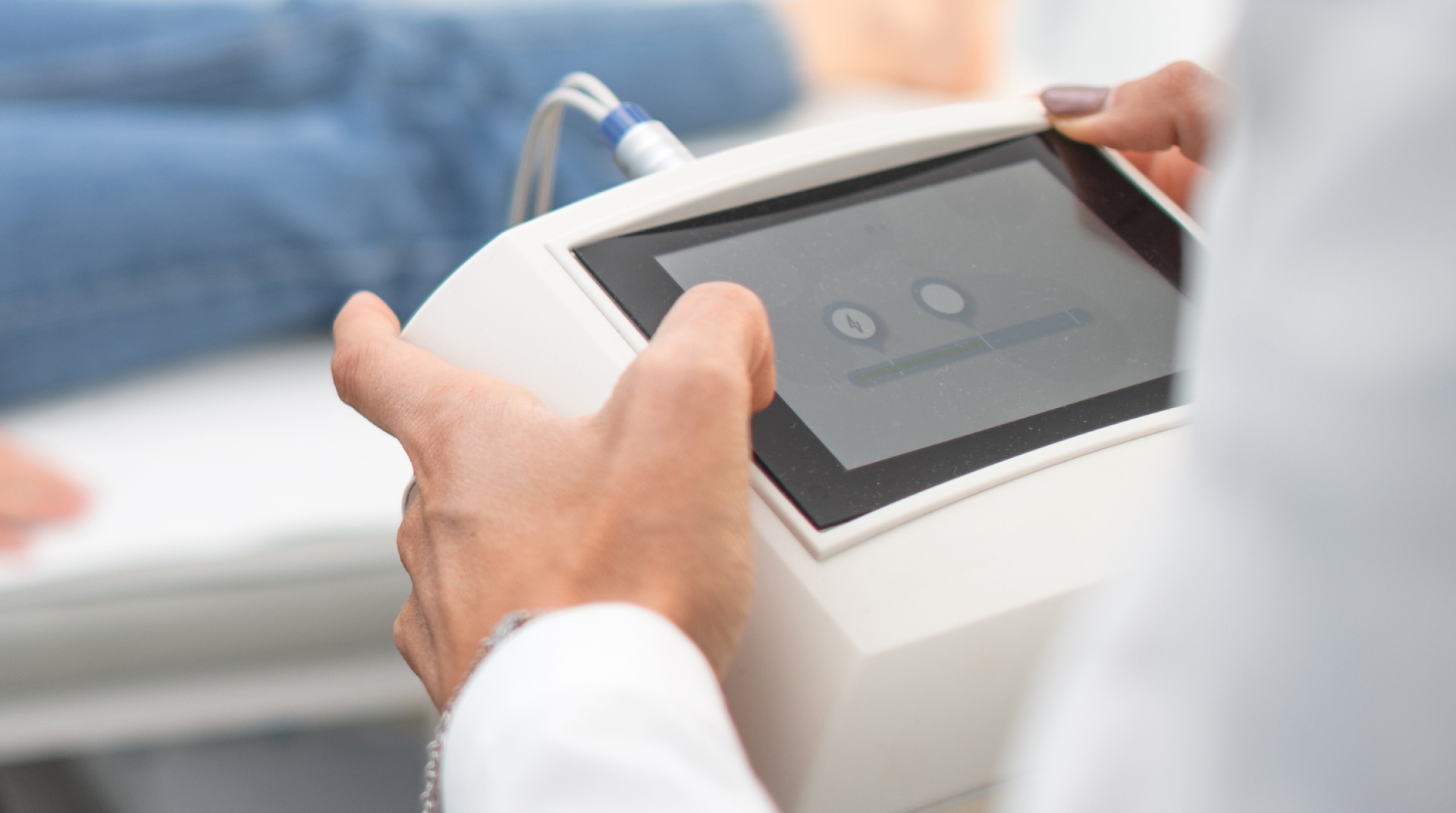
GlobalLogic DeviceSure
Transform the healthcare and patient experience with AI-powered, end-to-end testing for regulated medical devices and equipment
Accelerate medical device and equipment compliance and innovation
As regulatory pressures and market complexity increase, GlobalLogic’s DeviceSure solution empowers MedTech companies to meet stringent compliance standards while driving innovation. We streamline development with AI-powered test automation and comprehensive software assurance from chip to cloud. Engage with GlobalLogic to bring safe and effective medical devices to market, positioning your company at the forefront of medical technology.What We Offer
Comprehensive Compliance
GlobalLogic enables regulatory compliance with deep expertise in software assurance, helping MedTech companies achieve product approval and accelerate market entry.Enhanced Efficiency
GlobalLogic’s AI-driven test automation systems are architected for efficiency, streamlining product development, reducing time-to-market and allowing clients to innovate while maintaining quality.Cost Reduction
GlobalLogic reduces R&D costs with scalable, automated testing frameworks, saving time and resources while ensuring high-quality, compliant MedTech products.Cutting-edge Technology
GlobalLogic offers access to advanced technologies like Software-in-the-Loop/Hardware-in-the-Loop accelerators and cloud testing, ensuring devices are safe, effective, and submission-ready.Expertise and Partnerships
GlobalLogic’s industry expertise and strategic partnerships with test tool vendors and Hyperscalers provide MedTech companies with tailored solutions, driving innovation and leadership in healthcare.~40%increase in development efficiency~50%reduction in regression test time~11%project cost reductionEngineering healthcare impactOur real-world impact In action
Accelerating innovation in regulated industries
Fast-tracking Insulin Pump Release Cycles
Test automation for a hybrid, closed-loop insulin delivery system enables 15x faster verification testing and reduces manual effort by 400x
Accelerating Patient Monitoring System Testing
Automated testing to reduce effort by ~600x, cutting formal test cycles to two days with continuous malfunction detection and correction
Improving Neuro Implant Control Module Quality
HW/SW test automation solution for implants and tablets, achieving 100% automation including functionality, UI, and connectivity verification
Ensuring Robotic Guidance FDA Approval
Test automation framework ensured FDA readiness with 97% accuracy in control recognition by combining OpenCV and Machine Learning technologies
Featured insights
Explore fresh thinking from some of GlobalLogic’s strategists and engineers
See allBlogs25 April 2025Why Smarter Testing Is the Key to Winning the MedTech Innovation Race
DevOps-as-a-ServiceInnovation and Rapid PrototypingTesting-as-a-ServiceHealthcare and Life Sciences Blogs24 April 2025
Blogs24 April 2025GlobalLogic is your Trusted Partner for Semicon Growth in a Rapidly Evolving …
Semicon industry stakeholders can certainly agree that this is both…
Embedded Software, Hardware and Silicon SolutionsEnd-to-End IoT SolutionsInnovation and Rapid PrototypingSmart and Portable DevicesTesting-as-a-ServiceTechnology White Papers5 March 2025
White Papers5 March 2025Optimize MedTech Quality & Compliance with Automated Testing
Discover how automated testing accelerates MedTech innovation, enhances compliance, and…
AI-Powered SDLCTesting-as-a-ServiceHealthcare and Life SciencesWhy GlobalLogic?
GlobalLogic DeviceSure solutions drive meaningful business outcomes for our clients by addressing challenges in four key areas.

Deep Medical Domain Expertise
With 20+ years of experience and 3,000+ engineers, we develop FDA-approved and CE-marked products that meet international healthcare standards.
Regulated Product Development Process
We maintain an ISO 13485 certified quality system, harmonized with global standards, ensuring regulatory approvals across major markets.
Cross-Platform Digital Assurance
Our expertise spans real-time embedded systems, mobile, wearable devices, web/cloud apps, and AI models, including Software as a Medical Device (SaMDs).
Advanced Test Automation Expertise
We offer manual and automated testing, test strategy, planning, and analysis leveraging AI/ML-powered frameworks.
FAQsYour DeviceSure questions, answered
Learn more about DeviceSure at GlobalLogic.
-
How does GlobalLogic DeviceSure ensure compliance for medical devices?GlobalLogic DeviceSure combines deep medical domain expertise with advanced test automation to ensure regulatory compliance for medical devices and equipment. Our ISO 13485-certified quality system aligns with global standards, enabling clients to meet FDA and CE requirements while accelerating product approval and market entry.
-
What technologies power the DeviceSure solution?DeviceSure leverages AI-driven test automation, Software-in-the-Loop (SiL), Hardware-in-the-Loop (HiL) frameworks, and cloud-based testing tools to streamline medical device verification and validation. These cutting-edge technologies reduce testing time, enhance efficiency, and ensure safety and accuracy for both embedded systems and Software as a Medical Device (SaMD) applications.
-
How does DeviceSure reduce costs and improve efficiency for MedTech companies?DeviceSure’s scalable, automated testing frameworks minimize manual effort and streamline product development, leading to significant cost savings and efficiency gains. By automating regression tests, ensuring continuous malfunction detection, and reducing time-to-market, DeviceSure helps MedTech companies lower R&D expenses while maintaining the highest standards of quality and compliance.
Get in touchLet’s start engineering impact together.
GlobalLogic provides unique experience and expertise at the intersection of data, design, and engineering.

